Therac-25 Socio-technical Analysis
The safety of the Therac-25 is not really a property of the machine alone. Accidents that go unreported contribute to (or at least fail to stop) later accidents. When the TV camera in the room is unplugged, the operator cannot see that the patient is in trouble. So safety is really a property of the entire technical and social system (socio-technical system). In a similar manner, an ethical analysis of the issues in this case requires an awareness of the entire socio-technical system.
The safety of the Therac-25 is not really a property of the machine alone. Accidents that go unreported contribute to (or at least fail to stop) later accidents. When the TV camera in the room is unplugged, the operator cannot see that the patient is in trouble. So safety is really a property of the entire technical and social system (socio-technical system). In a similar manner, an ethical analysis of the issues in this case requires an awareness of the entire socio-technical system.
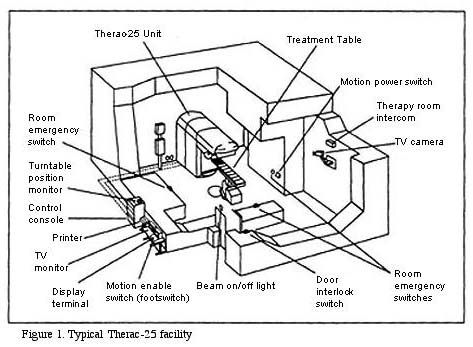
The Therac-25 Medical Linear Accelerator is a large machine that sits in a room designed just for it. We think of the machine itself or the machine-in-the-room as the system. But the larger system, or the Socio-Technical system, that we need to think about includes:
- Hardware: The mechanics of the machine itself, including its associated computer
- Software: the operating system of the computer and the operating system of the machine
- Physical surroundings: the room with its shielding, cameras, locking doors, etc.
- People: operators, medical physicists, doctors, engineers, salespeople, managers at AECL, government regulators
- Institutions: AECL, FDA, each medical facility, associations of operators, etc.
- Procedures
- Management models: AECL’s model of how risk is managed
- Reporting relationships: who was required to report accidents to whom
- Documentation requirements: for the software, for the facilities, for the FDA
- Data flow: how different parts of AECL shared information, how information was shared among agencies and organizations, how data was used by the Therac software.
- Rules & norms: what patients are "normally" told, what operator & physicist responsibilities are, expectations set for the programmer
- Laws and regulations: Reporting requirements, FDA enforcement mechanisms, medical liability law
- Data: data was collected in FDA approval process, use of data in Therac software,
The following table presents some of these items in a schematic form.
|
The Socio-Technical System |
|
|
The Machine
|
Hospitals and Clinics
|
|
Atomic Energy Canada, Limited
|
Government Medical Device Regulation
|
A thorough investigation of the Therac-25 case requires some grasp of most of these items. You will come across most of these items as you read this case. Setting your sights on the entire system will help you avoid the trap of finding a single point of blame. It is easy, for instance, to decide that the programmer made serious mistakes and to end one’s analysis there. This is a short-sighted approach. It would miss the problems with maintenance in the cancer therapy facilities; it would miss the incomplete reporting requirements for the FDA; it would miss the inadequate and misleading testing of the Therac-25 system.
Material originally from ComputingCases.org developed by Dr. Charles Huff of St. Olaf College.